This site is intended for United Kingdom/Ireland healthcare professionals
Vyxeos Liposomal is indicated for the treatment of adults with newly-diagnosed t-AML or AML-MRC1
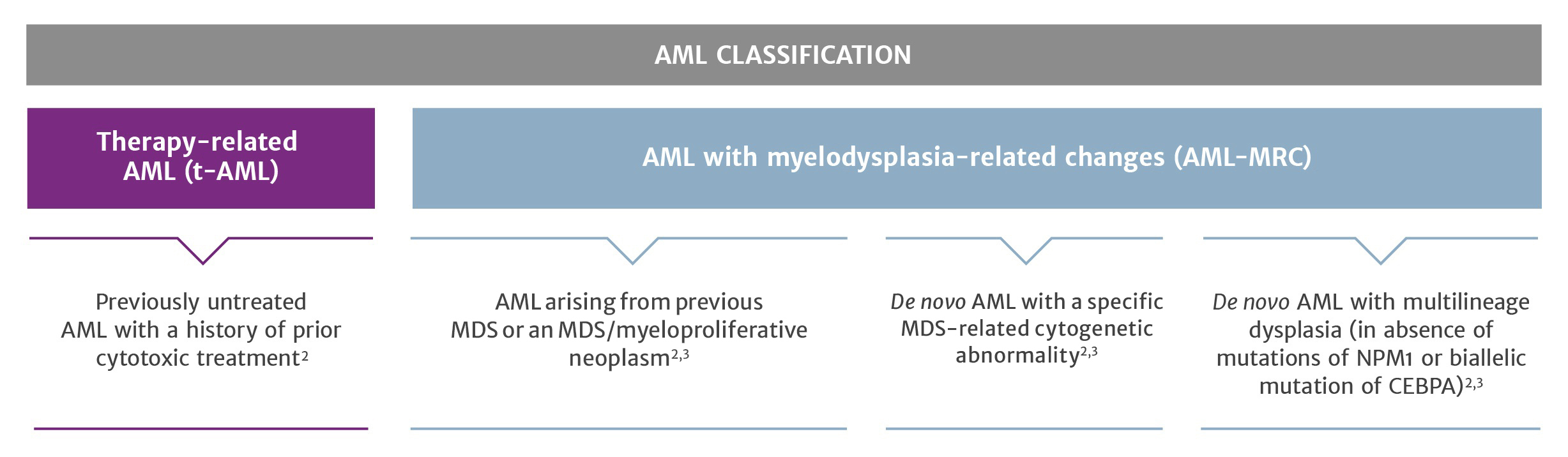
- The World Health Organisation uses clinical features, morphology, cytogenetics and molecular genetics to define AML disease entities of clinical significance2
- t-AML and AML-MRC are distinct subcategories with high-risk features4
- Over a quarter of AML patients may fall into these high-risk categories5,6
Vyxeos Liposomal Reimbursement Status in the United Kingdom
![]()
National Institute for Health & Care Excellence (NICE) guidance8
Liposomal cytarabine–daunorubicin is recommended, within its marketing authorisation, as an option for untreated t-AML or AML-MRC in adults. It is recommended only if the company provides it according to the commercial arrangement8
![]()
Scottish Medicines Consortium (SMC) advice9
Advice: Following a full submission assessed under the end of life and ultra-orphan medicine process, liposomal formulation of daunorubicin/cytarabine (Vyxeos Liposomal) is accepted for use within NHS Scotland9
Indication under review: The treatment of adults with newly-diagnosed t-AML or AML-MRC. In a randomised Phase 3 study, in adults (aged 60 to 75 years) with high-risk* AML, liposomal daunorubicin/cytarabine improved overall survival when compared with a standard of care regimen9
This SMC advice takes account of the benefits of a Patient Access Scheme (PAS) that improves the cost-effectiveness of liposomal daunorubicin/cytarabine. This advice is contingent upon the continuing availability of the PAS in NHS Scotland or a list price that is equivalent or lower9

Health Service Executive (HSE) approval10,11
In February 2021, the HSE approved for reimbursement Vyxeos Liposomal 44 mg/100 mg powder for concentrate for solution for infusion (daunorubicin and cytarabine): “For the treatment of adults with newly diagnosed, therapy-related acute myeloid leukaemia (t-AML) or AML with myelodysplasia-related changes (AML-MRC)”10,11
Vyxeos Liposomal: An Innovative Mode of Action1, 12-14
Vyxeos Liposomal is the first dual-drug advanced liposomal formulation of daunorubicin and cytarabine designed to optimise efficacy of treatment in high-risk AML12,13
Synergistic Ratio
Fixed 1:5 molar ratio of DA within an advanced liposomal formulation1
Prolonged Bioavailability
Synergistic molar ratio maintained for a prolonged period of time – over 24 hours after administrations1,14
High Concentration
Vyxeos Liposomal accumulates and persists in the bone marrow in high concentrations in animal models1
Preferential Uptake
Vyxeos Liposomal is preferentially taken up by leukaemia cells vs. normal bone marrow cells in leukaemia-bearing mice1